Health Lab
Michigan Medicine's daily online publication featuring news and stories about the future of health care.

Health Lab
Most human oocytes never get a chance to mature into eggs — a new study sheds light on why

Want top health & research news weekly? Sign up for Health Lab’s newsletters today!
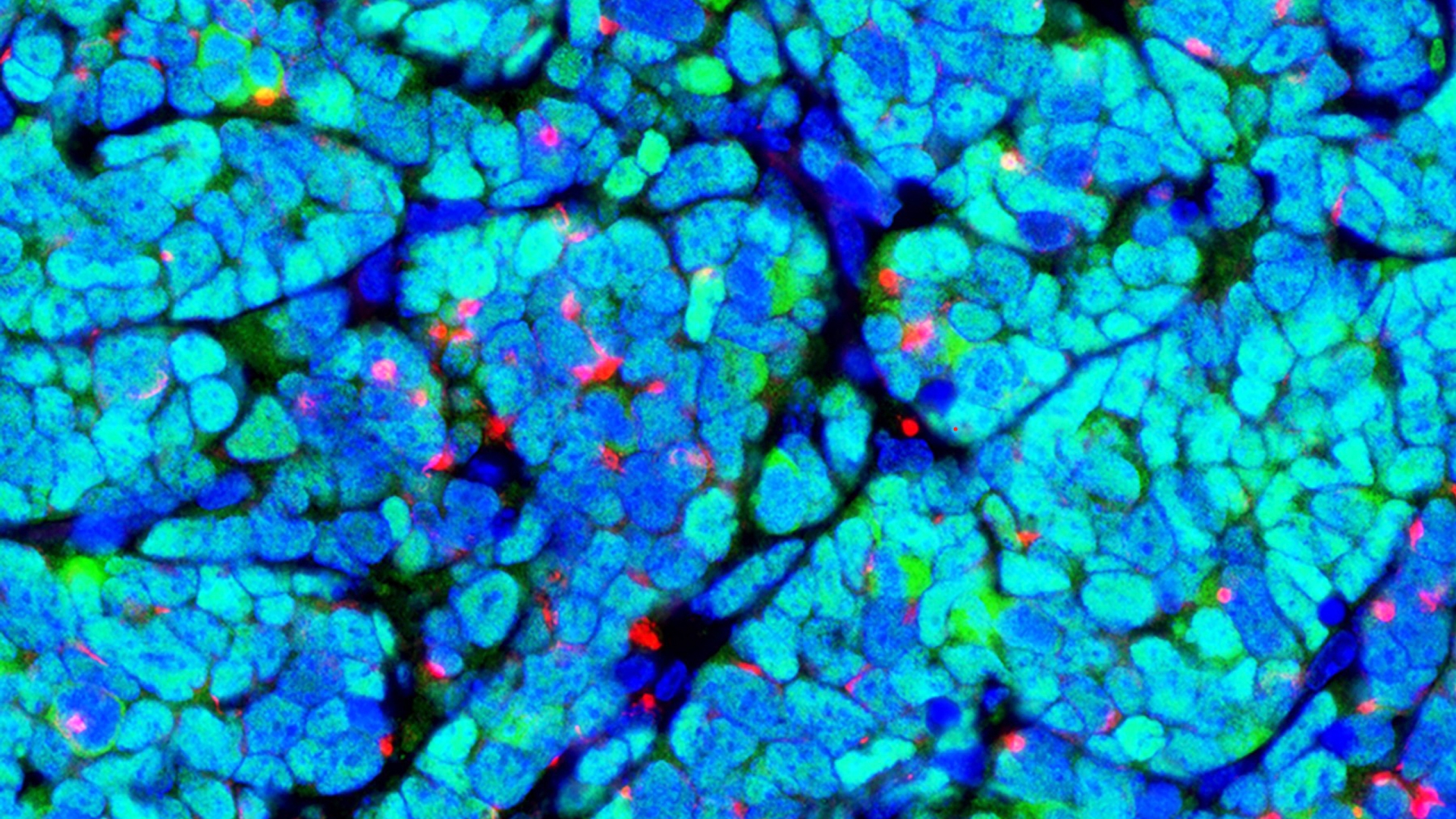
Health Lab
New map of the ovary provides a deeper understanding of how oocytes interact with the surrounding cells during the normal maturation process, and how the function of the follicles may break down in aging or fertility related diseases.

Health Lab
Among people with multiple sclerosis in the United States, more than half experienced at least one fall in a six-month period and approximately one-third of those falls resulted in an injury.

Health Lab
An expert from the University of Michigan Addiction Center shares the impacts of teen substance use and what families can do to help youth who may be at risk or showing signs of addiction.

Health Lab
After a series of life altering health setbacks following a devastating crash, Gabe Villanueva’s is on an extraordinary journey of survival thanks to the highly skilled team at University of Michigan Health.
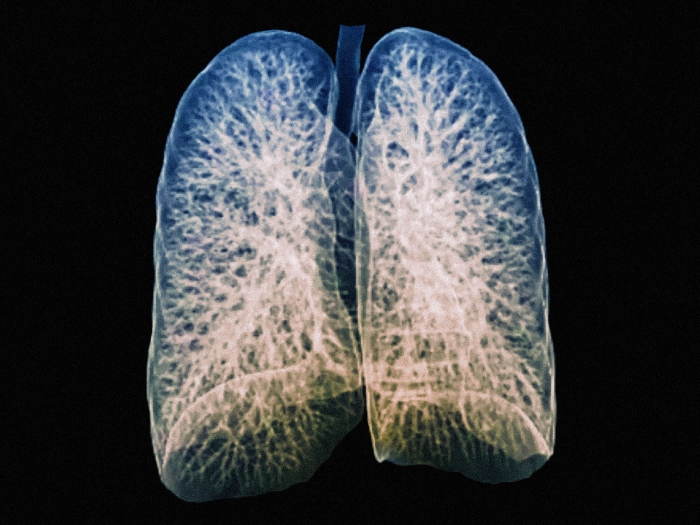
Health Lab
A recent U-M study uncovers a pathway utilized during normal wound healing that has the potential to reverse idiopathic pulmonary fibrosis.

Health Lab
Survey shows many teens and young adults support making menstrual products more accessible to fight "period poverty."

The Health Lab Podcast
Subscribe today on Apple Podcasts, Google Podcasts, Spotify, Stitcher or wherever you get your podcasts.

Health Lab
Among people with multiple sclerosis in the United States, more than half experienced at least one fall in a six-month period and approximately one-third of those falls resulted in an injury.

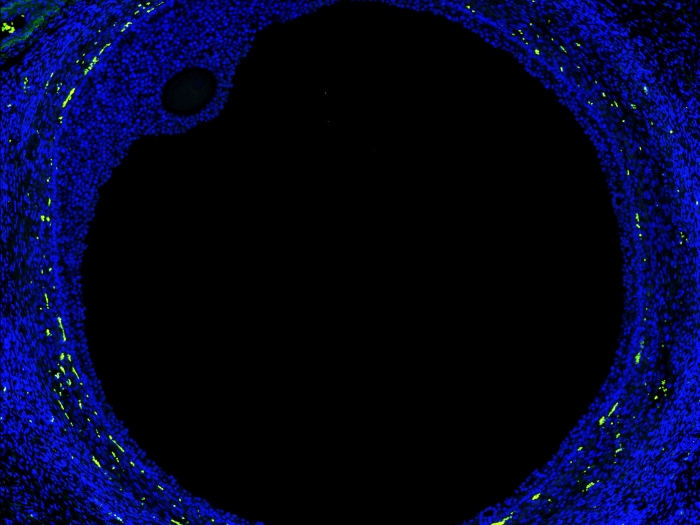
Health Lab
New map of the ovary provides a deeper understanding of how oocytes interact with the surrounding cells during the normal maturation process, and how the function of the follicles may break down in aging or fertility related diseases.

Health Lab
A recent U-M study uncovers a pathway utilized during normal wound healing that has the potential to reverse idiopathic pulmonary fibrosis.

Health Lab
Survey shows many teens and young adults support making menstrual products more accessible to fight "period poverty."
Health Lab
An effort to reduce use of PPI heartburn drugs in veterans because of overuse, cost and potential risks succeeded, but provides lessons about deprescribing efforts.

Health Lab
In the past two years, 60% of people age 50 to 80 have visited an urgent care clinic, or a clinic based in a retail store, workplace or vehicle, according to new findings from the University of Michigan National Poll on Healthy Aging.