Health Lab
Michigan Medicine's daily online publication featuring news and stories about the future of health care.

Health Lab
Discovery raises hope for more targeted approaches to treatment of autoimmune blood clotting disorder

Want top health & research news weekly? Sign up for Health Lab’s newsletters today!

Health Lab
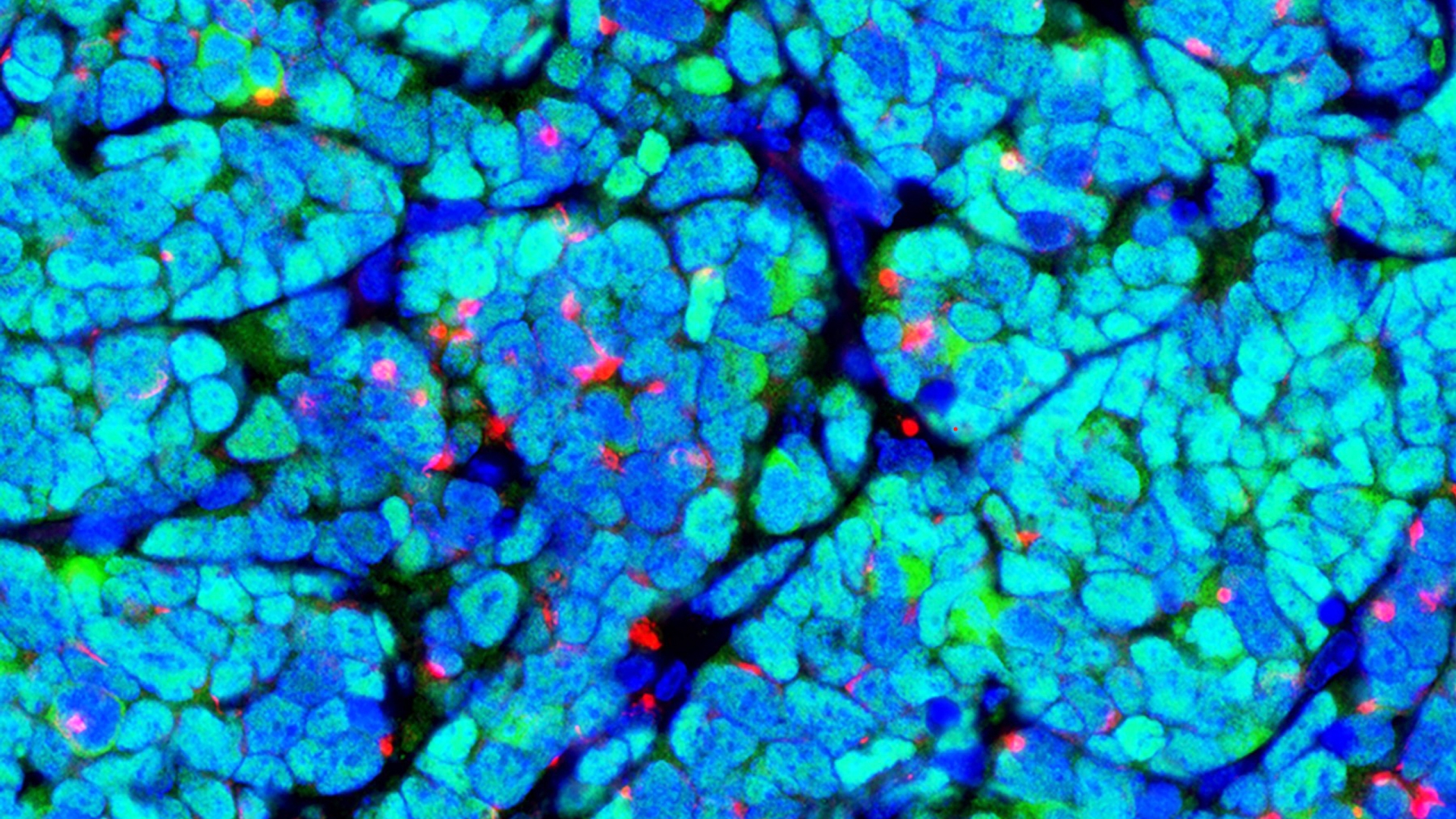
Researchers recently revealed a new mechanism behind antiphospholipid syndrome that the investigators hope will eventually allow treatments to be targeted closer to the source of the problem.

Health Lab
Data on medical cannabis use found that enrollment in medical cannabis programs increased overall between 2016 and 2022, but enrollment in states where nonmedical use of cannabis became legal saw a decrease in enrollment

Health Lab
A new urine-based test addresses a major problem in prostate cancer: how to separate the slow growing form of the disease unlikely to cause harm from more aggressive cancer that needs immediate treatment.
Health Lab
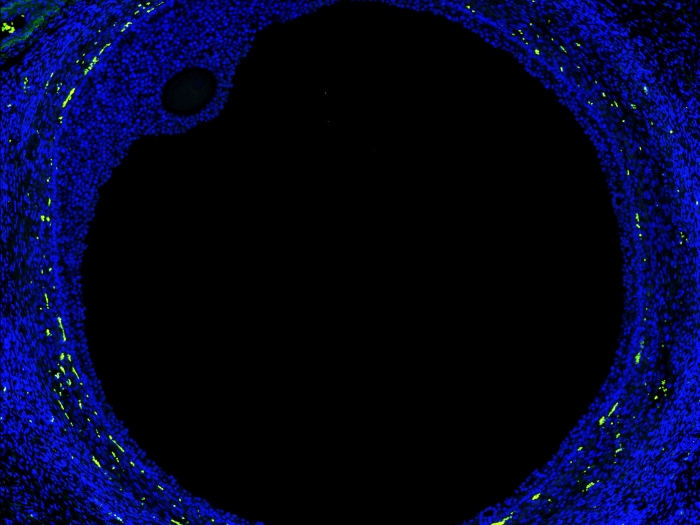
New map of the ovary provides a deeper understanding of how oocytes interact with the surrounding cells during the normal maturation process, and how the function of the follicles may break down in aging or fertility related diseases.

Health Lab
Among people with multiple sclerosis in the United States, more than half experienced at least one fall in a six-month period and approximately one-third of those falls resulted in an injury.

Health Lab
An expert from the University of Michigan Addiction Center shares the impacts of teen substance use and what families can do to help youth who may be at risk or showing signs of addiction.

The Health Lab Podcast
Subscribe today on Apple Podcasts, Google Podcasts, Spotify, Stitcher or wherever you get your podcasts.

Health Lab
Data on medical cannabis use found that enrollment in medical cannabis programs increased overall between 2016 and 2022, but enrollment in states where nonmedical use of cannabis became legal saw a decrease in enrollment

Health Lab
A new urine-based test addresses a major problem in prostate cancer: how to separate the slow growing form of the disease unlikely to cause harm from more aggressive cancer that needs immediate treatment.

Health Lab
Researchers recently revealed a new mechanism behind antiphospholipid syndrome that the investigators hope will eventually allow treatments to be targeted closer to the source of the problem.

Health Lab
Among people with multiple sclerosis in the United States, more than half experienced at least one fall in a six-month period and approximately one-third of those falls resulted in an injury.

Health Lab
New map of the ovary provides a deeper understanding of how oocytes interact with the surrounding cells during the normal maturation process, and how the function of the follicles may break down in aging or fertility related diseases.
Health Lab
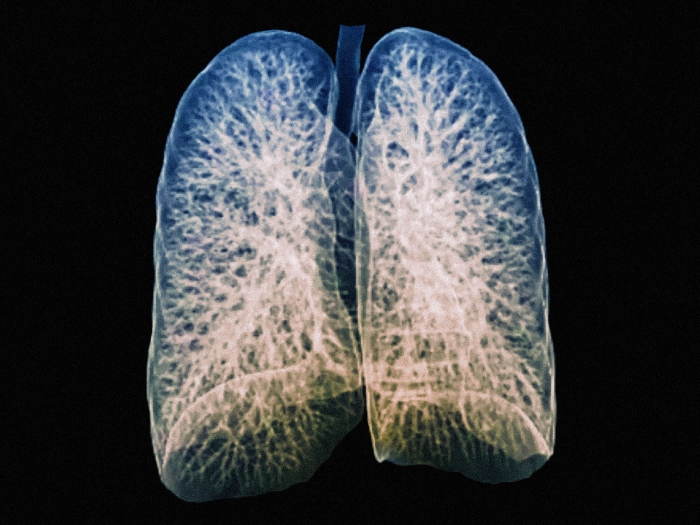
A recent U-M study uncovers a pathway utilized during normal wound healing that has the potential to reverse idiopathic pulmonary fibrosis.