Health Lab
Michigan Medicine's daily online publication featuring news and stories about the future of health care.

Health Lab
An end-of-life care specialist reflects on how Medicare’s regulations for enrolling in hospice exclude many dementia patients who need it the most

Want top health & research news weekly? Sign up for Health Lab’s newsletters today!

Health Lab
An end-of-life care specialist discusses the shortfalls of hospice care coverage for people with dementia, using the experience of former President Jimmy Carter and former First Lady Rosalynn Carter as examples.
Health Lab
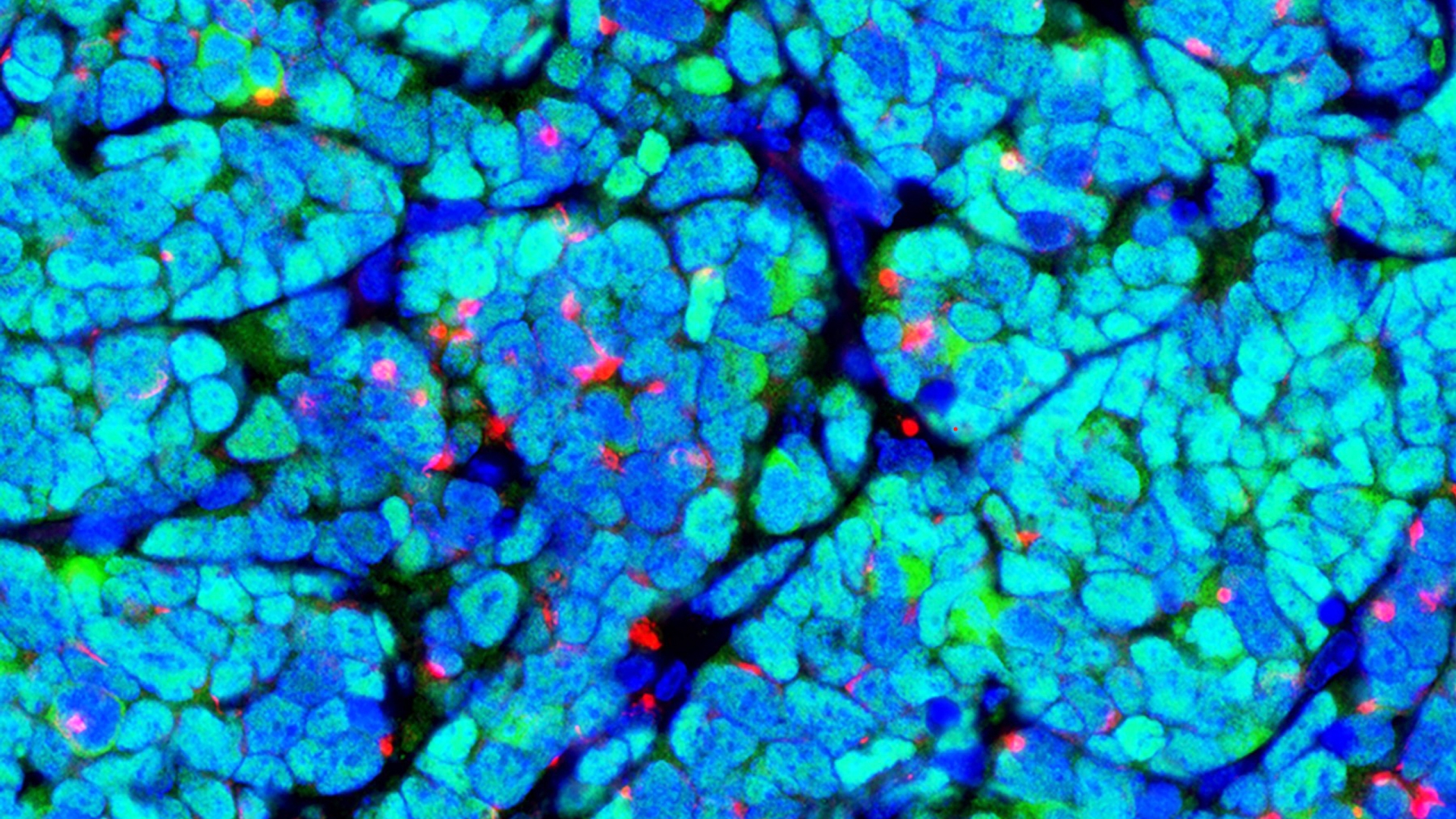
Researchers improved memory and reduced neuroinflammation in a mouse model of Alzheimer’s Disease, suggesting another avenue for potential treatment.

Health Lab
Malinda and David Morrison III welcomed their son in 2022 after years of trying to conceive
Health Lab
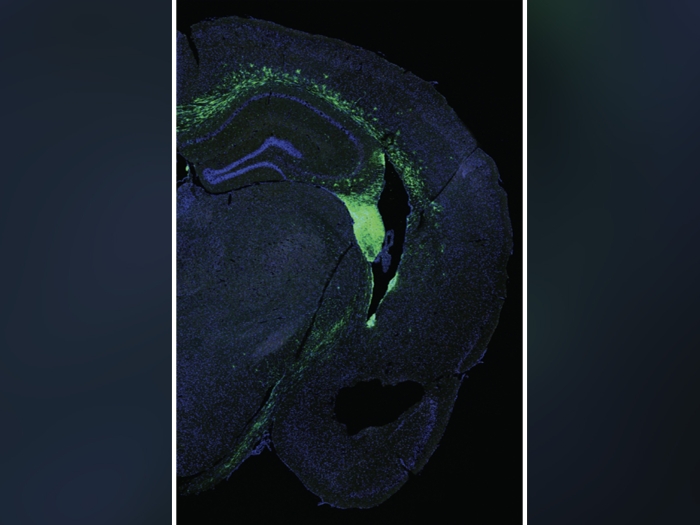
Buprenorphine prescribing for opioid addiction used to require a special waiver from the federal government, but a new study shows what happened in the first year after that requirement was lifted.

Health Lab
Researchers have used advanced computer algorithms to uncover distinct molecular subgroups of kidney diseases, independent of clinical classifications. These findings have significant implications for personalized treatment approaches.

Health Lab
Young heart transplant recipient develops post-transplant lymphoma, but perseveres

The Health Lab Podcast
Subscribe today on Apple Podcasts, Google Podcasts, Spotify, Stitcher or wherever you get your podcasts.

Health Lab
Researchers improved memory and reduced neuroinflammation in a mouse model of Alzheimer’s Disease, suggesting another avenue for potential treatment.

Health Lab
Buprenorphine prescribing for opioid addiction used to require a special waiver from the federal government, but a new study shows what happened in the first year after that requirement was lifted.

Health Lab
Researchers have used advanced computer algorithms to uncover distinct molecular subgroups of kidney diseases, independent of clinical classifications. These findings have significant implications for personalized treatment approaches.
Health Lab
Overuse of antibiotics can lead bacteria to evolve antimicrobial resistance, but Americans are still receiving the drugs for many conditions that they can’t treat.

Health Lab
Data on medical cannabis use found that enrollment in medical cannabis programs increased overall between 2016 and 2022, but enrollment in states where nonmedical use of cannabis became legal saw a decrease in enrollment

Health Lab
A new urine-based test addresses a major problem in prostate cancer: how to separate the slow growing form of the disease unlikely to cause harm from more aggressive cancer that needs immediate treatment.